Background
Those familiar with the HIIT Science material, know the issue all too well. There is the training load an athlete encounters, which we have some level of control over. But then there is the training load response — a completely different beast. This issue, and the problem it can cause, has frustrated athletes, teams and coaches for years.
Why on earth is the response to training so variable?
Recently, we had the opportunity to collaborate with a large group of colleagues on some projects aiming to understand more about the individual responses to training, in the hope of learning how we can individualize training prescription more effectively, particularly for athletes. To be clear, if two athletes perform the same type of training session or training block, it is unlikely that the athletes will have the same acute response or adaptation, nor will they perceive the training stimulus in exactly the same way. There are a multitude of reasons for these divergent responses that we find very intriguing.
One factor that may explain some degree of these variable responses is the variability in the ratio of type I (slow-twitch) and II (fast-twitch) muscle fibres (i.e., muscle fibre typology). Type I muscle fibres produce force relatively slowly (6) but have superior fatigue resistance compared to type II fibres (10, 27). As illustrated simplistically, Type II fibres can produce more power (6), but are thought to have greater fatigability than type I fibres (29).

Predominant muscle fibre type in an athlete affects their ability to produce speed/power.
It is conceivable that variation in the proportion of these fibres may dictate how an athlete responds to training from an acute recovery time course perspective, as well as the long-term chronic adaptations to a given training block.
Is muscle fibre type typology important for sports performance?
Classical studies from the 1970’s and 1980’s (8, 9, 13, 14, 17, 25) showed that muscle fibre typology differentiated between athletes specializing in different sport disciplines, as well as the competitive level of athletes within these disciplines. For example, international level distance runners possessed a significantly greater percentage of type I fibres (mean; range: 69; 63-74%) in the gastrocnemius compared to middle-distance (61; 44-73%) and sprint-distance runners (27; 27-28%) (9).
Other studies show that athletes with a higher proportion of type II fibres typically have superior sprint performance, but have greater fatigability during and a longer time course of recovery following high-intensity exercise compared to those athletes with a higher proportion of type I fibres (15, 16, 19). As such, variation in muscle fibre typology might be important for specializing in different sports and for individualizing training and recovery cycles.
Classifying muscle fibre types
Muscle fibres have traditionally been classified by analyses of their myosin heavy chain (MHC) isoforms and can be identified as pure (i.e., type I, IIa, IIx) or hybrid fibres that co-express two or more MHC isoforms (i.e., I/IIa, I/IIa/IIx, IIa/IIx, I/IIx) (6, 30).

Normalized power was 2-, 6-, and 12-fold greater in MHC I/IIa, MHC IIa, and MHC IIa/IIx fibres, respectively, when compared with MHC I fibres. (Galpin et al. (12)).
One of the major reasons that muscle fibre typology is not routinely measured to guide sport and discipline specialisation and training advice for athletes, is that the muscle biopsy technique is typically met with resistance from athletes given that it is highly invasive and disruptive to training. While muscle biopsies allow for the direct determination of MHC composition, the disadvantage is the low test-retest reliability (5, 26, 28), which may result from alterations in the depth of the muscle biopsy (20, 22), the yield of fibres that are obtained (24) and/or other sources of technical error (28). As such, a valid and reliable non-invasive technique to estimate muscle fibre typology would circumvent some of these limitations and allow us to apply the information that we’ve learned from classical muscle biopsy studies for talent identification and training prescription for athletes.
The Muscle Talent Scan
Nearly ten years ago, Professor Wim Derave’s lab of Ghent University developed a non-invasive method to estimate muscle fibre typology, based on the measurement of muscle carnosine by proton magnetic resonance spectroscopy (1H-MRS) (1).
Carnosine is a stable, dipeptide that is present in two-fold higher concentrations in type II fibres compared to type I fibres (7, 18) and has a strong positive association with the muscle biopsy determined percentage of total area occupied by type II fibres in males (r = 0.714, P = 0.009, n = 12 males) (1). 1H-MRS allows for the measurement of an absolute carnosine concentration and this value is expressed as a Z-score relative to a non-athlete control population that have previously been scanned (1, 2).
We do this so that both men and women can be integrated into the same analysis given that women consistently show lower levels of muscle carnosine (11, 23), which may be mutually exclusive from any potential sex-based difference in muscle fibre typology. Studies that we have conducted at Ghent University (Belgium) and at Griffith University (Gold Coast, Australia) have shown extremely high levels of reliability between inter-day 1H-MRS measurements of muscle carnosine (coefficient of variation: 3 – 8%).
While the 1H-MRS measurement of muscle carnosine is an estimation of muscle fibre typology, this technique has the advantage of sampling a substantially larger volume of muscle compared to the muscle biopsy technique (1), which better accounts for the variation in muscle fibre typology in the superficial and deep parts of a given muscle (20, 22).
Furthermore, the major advantage of the 1H-MRS measurement of muscle fibre typology is the non-invasive nature, making this much more palatable for elite athletes.

Absolute carnosine concentration is expressed as a Z-score relative to a non-athlete control population that have previously been scanned. The carnosine Z-score provides an estimation of the muscle fibre typology of an athlete. (Original figure, Prof Derave’s Lab).
The research
Athlete characterization
Our first studies showed that the estimation of muscle fibre typology with 1H-MRS provides a systematic distinction in the estimated muscle fibre typology between sprint- and endurance-type athletes both within and between different sports (1, 3, 4).
Acute responses to training
In the first of our experimental studies (21), we aimed to determine whether recreationally active athletes with a higher estimated proportion of type II fibres (carnosine Z-score >0.5) had greater fatigue during a sprint-interval training session as well as delayed recovery in the subsequent 5 h, compared to those subjects with a higher estimated proportion of type I fibres (carnosine Z-score <-0.5).
The thought behind this study came from our observations of training sessions with groups of athletes who, despite giving the same perceived effort, recovered more quickly between repetitions, while others required longer recovery times to run the same time or produce the same power output as the previous repetition.
In this study, both groups produced the same mean power output across the sprint-interval training session; however, the drop in power output throughout the sprint intervals was higher in the group of subjects with more type II fibres (-61%) compared to the group with more type I fibres (-41%). The torque at maximal voluntary contraction had fully recovered in the latter group after 20 min, while the subjects with more type II fibres had not yet recovered 5 h into recovery.
We interpret these findings to suggest that only 90 seconds of all-out sprint exercise can induce long-lasting fatigue and impairments in the contractile function of the muscles of subjects with more type II fibres and resulted in incomplete recovery up to 5 h post sprint training.
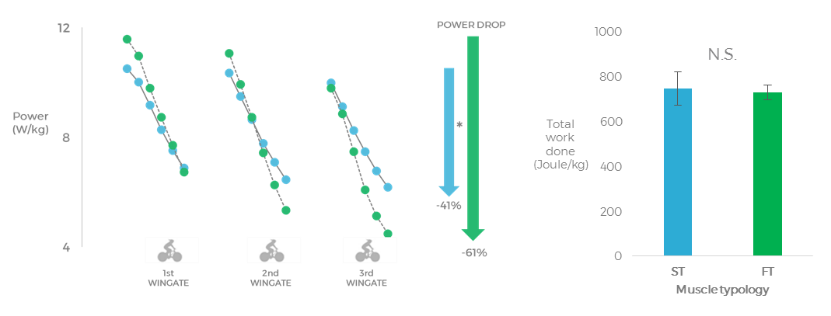

The left figure shows the power output during the 3 Wingates, the group with a higher estimated proportion of type II fibres displayed a larger power drop when compared to the group with a higher estimated proportion of type I fibres. The middle figure shows that the total work done during the sprints was the same in both groups. The right figure shows that the group with a higher estimated proportion of type I fibres was able to recover maximal force from the sprint-interval session in 20 min, while the group with a higher estimated proportion of type II fibres was not yet recovered after 5 hours. (Lievens et al. (21)).
Practical applications
- The 1H-MRS measurement of muscle fibre typology can be used to categorise athletes into sports and/or disciplines that are fast and explosive or slow and endurance-based.
- While coaches can use various performance measures to estimate the muscle fibre typology in their athletes, most of these estimation methods are responsive to training. Carnosine is a stable intramuscular metabolite that is largely independent of extrinsic factors, such as diet (except for beta-alanine supplementation) and training, and its concentration is markedly different between type I and II fibres. This makes it a good candidate for the estimation of muscle fibre typology.
- The acute recovery time following a sprint interval training session is dependent upon an individual’s estimated muscle fibre typology, which has implications for how training sessions are distributed across a weekly program.
- Athletes with a greater estimated proportion of type II fibres had a 20% higher drop in power output during subsequent sprints within a HIIT session, which has implications for session design given that the work interval intensity is a key training prescription variable.
- The distribution of hard interval sessions within a given training week could be a major consideration for athletes with a greater estimated proportion of type II fibres given that their recovery between sessions is considerably slower compared to athletes with a greater estimated proportion of type I fibres.
Conclusion
We found these findings intriguing, and with such polarized responses in the acute recovery time course following a single sprint-interval training session, we wondered whether we would also see divergent responses across more chronic training periods. In the follow-up blog, we will discuss the findings from these projects.
Phil Bellinger & Eline Lievens
This is an article of HIIT Science, it was published on their website.
About the authors

Eline Lievens is a PhD student at the Department of Movement and Sports Sciences Ghent University (Ghent, Belgium)
Email: elilieve.lievens@ugent.be

Dr Phil Bellinger is a lecturer at Griffith University (Gold Coast, Australia)
Email: p.bellinger@griffith.edu.au
References
- Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, and Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLOS ONE 6: e21956, 2011.
- Bellinger P, Desbrow B, Derave W, Lievens E, Irwin C, Sabapathy S, Kennedy B, Craven J, Pennell E, Rice H, and Minahan C. Muscle fiber typology is associated with the incidence of overreaching in response to overload training. Journal of Applied Physiology doi.org/10.1152/japplphysiol.00314.2020
- Bex T, Baguet A, Achten E, Aerts P, De Clercq D, and Derave W. Cyclic movement frequency is associated with muscle typology in athletes. Scandinavian journal of medicine & science in sports 27: 223-229, 2017.
- Bex T, Iannaccone F, Stautemas J, Baguet A, De Beule M, Verhegghe B, Aerts P, De Clercq D, and Derave W. Discriminant musculo‐skeletal leg characteristics between sprint and endurance elite Caucasian runners. Scandinavian journal of medicine & science in sports 27: 275-281, 2017.
- Blomstrand E and Ekblom B. The needle biopsy technique for fibre type determination in human skeletal muscle–a methodological study. Acta Physiologica Scandinavica 116: 437-442, 1982.
- Bottinelli R, Canepari M, Pellegrino MA, and Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. The Journal of Physiology 495: 573-586, 1996.
- C. Harris R, Dunnett M, and Greenhaff PL. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. Journal of Sports Sciences 16: 639-643, 1998.
- Costill D, Fink W, and Pollock M. Muscle fiber composition and enzyme activities of elite distance runners. Medicine and science in sports 8: 96-100, 1976.
- Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, and Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. Journal of Applied Physiology 40: 149-154, 1976.
- Essén B, Jansson E, Henriksson J, Taylor AW, and Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiologica Scandinavica 95: 153-165, 1975.
- Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, Taes Y, De Heer E, and Derave W. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids 40: 1221-1229, 2011.
- Galpin AJ, Raue U, Jemiolo B, Trappe TA, Harber MP, Minchev K, and Trappe S. Human skeletal muscle fiber type specific protein content. Analytical Biochemistry 425: 175-182, 2012.
- Gerard ES, Caiozzo VJ, Rubin BD, Prietto CA, and Davidson DM. Skeletal muscle profiles amon elite long, middle, and short distance swimmers. The American Journal of Sports Medicine 14: 77-82, 1986.
- Gollnick P, Armstrong R, Saubert C, Piehl K, and Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. Journal of Applied Physiology 33: 312-319, 1972.
- HÄkkinen K and Komi PV. Effects of fatigue and recovery on electromyographic and isometric force- and relaxation-time characteristics of human skeletal muscle. European Journal of Applied Physiology and Occupational Physiology 55: 588-596, 1986.
- Hamada T, Sale DG, MacDougall JD, and Tarnopolsky MA. Interaction of fibre type, potentiation and fatigue in human knee extensor muscles. Acta Physiologica Scandinavica 178: 165-173, 2003.
- Houston M, Wilson D, Green H, Thomson J, and Ranney D. Physiological and muscle enzyme adaptations to two different intensities of swim training. European Journal of Applied Physiology and Occupational Physiology 46: 283-291, 1981.
- Kendrick IP, Kim HJ, Harris RC, Kim CK, Dang VH, Lam TQ, Bui TT, and Wise JA. The effect of 4 weeks β-alanine supplementation and isokinetic training on carnosine concentrations in type I and II human skeletal muscle fibres. European Journal of Applied Physiology 106: 131-138, 2009.
- Komi PV and Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. European Journal of Applied Physiology and Occupational Physiology 42: 41-50, 1979.
- Lexell J, Henriksson-Larsen K, and Sjöström M. Distribution of different fibre types in human skeletal muscles 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiologica Scandinavica 117: 115-122, 1983.
- Lievens E, Klass M, Bex T, and Derave W. Muscle fiber typology substantially influences time to recover from high-intensity exercise. Journal of Applied Physiology 128: 648-659, 2020.
- Mahon M, Toman A, Willan PLT, and Bagnall KM. Variability of histochemical and morphometric data from needle biopsy specimens of human quadriceps femoris muscle. Journal of the Neurological Sciences 63: 85-100, 1984.
- Mannion AF, Jakeman PM, Dunnett M, Harris RC, and Willan PLT. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. European Journal of Applied Physiology and Occupational Physiology 64: 47-50, 1992.
- Nederveen JP, Ibrahim G, Fortino SA, Snijders T, Kumbhare D, and Parise G. Variability in skeletal muscle fibre characteristics during repeated muscle biopsy sampling in human vastus lateralis. Applied Physiology, Nutrition, and Metabolism 45: 368-375, 2020.
- Nygaard E. Skeletal muscle fibre characteristics in young women. Acta Physiologica Scandinavica 112: 299-304, 1981.
- Nygaard E and Sanchez J. Intramuscular variation of fiber types in the brachial biceps and the lateral vastus muscles of elderly men: How representative is a small biopsy sample? The Anatomical Record 203: 451-459, 1982.
- Prince FP, Hikida RS, Hagerman FC, Staron RS, and Allen WH. A morphometric analysis of human muscle fibers with relation to fiber types and adaptations to exercise. Journal of the Neurological Sciences 49: 165-179, 1981.
- Sahl RE, Morville T, Kraunsøe R, Dela F, Helge JW, and Larsen S. Variation in mitochondrial respiratory capacity and myosin heavy chain composition in repeated muscle biopsies. Analytical Biochemistry 556: 119-124, 2018.
- Schiaffino S and Reggiani C. Fiber types in mammalian skeletal muscles. Physiological Reviews 91: 1447-1531, 2011.
- Stienen GJ, Kiers JL, Bottinelli R, and Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre type and temperature dependence. The Journal of Physiology 493: 299-307, 1996.





